Let the other universities brand themselves with the presidents they’ve produced, the corporations they’ve midwifed, their location in a small town outside of Boston, or their number one football team.
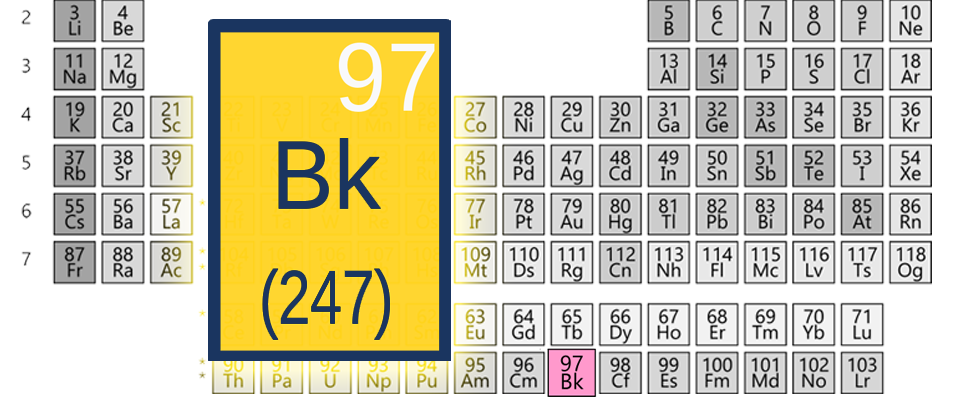
At Berkeley, we’re OK with being number 97. On the periodic table of elements. You may have heard of “the table,” as we call it around here. It’s sort of the ingredients list for the universe. All of it, including presidents, corporations, slushy college towns, and inferior (spiritually) football teams.
Yup. Good ol‘ 97, berkelium, Bk for short. Or you could look us up under number 98, californium. Mind you, we didn’t name these elements after ourselves until after we had graciously bestowed like honor on an entire continent or two when our researchers synthesized and named number 95, americium. Modesty, you know.
And who could forget the most exciting element Berkeley introduced to the world, number 94, plutonium? Really, it’s probable that no other university has done as much to threaten the existence of human civilization. Sorry, Yale.
And then there’s seaborgium, lawrencium, astatine, and on and on. Berkeley and Lawrence Berkeley National Laboratory can claim 16 elements. That’s about 14 percent of the known periodic table that’s ours, indisputably.
Well, maybe not indisputably….
We speak here of the great and terrible Transfermium Wars, the only atomic wars the United States ever fought with the Soviet Union, wars of such animosity that they outlasted the existence of the Soviet Union by several years. Happily for the rest of the world, the radiation was confined to the labs and the war was fought with words.
Prior to 1957, the discovery and naming of new atomic elements was easy: Berkeley discovered them and named them. All the way up to 100, fermium, and 101, mendelevium.
In 1957, however, some upstarts with a cyclotron at the Nobel Institute in Sweden claimed to have discovered 102, which they named nobelium. In 1958, a Berkeley team led by Albert Ghiorso fired up its cyclotron, and couldn’t replicate the Swedish results but found another isotope of 102 and said, for the sake of clarity, let’s keep calling it nobelium.
Meanwhile, at the Joint Institute for Nuclear Research lab in the Soviet Union, Georgy Flerov was leading a team of scientists with their own atom smasher. Flerov’s team would claim to have discovered nobelium in 1956 (but not to have published their results) and to have synthesized it again in 1958.
On and on it went through the ’60s and ’70s, with both labs synthesizing elements 103, 104, 105, and 106, and both labs challenging each other’s results. Finally, in 1986, the International Union of Pure and Applied Chemistry created a panel of scientists from neither country to look into who discovered what and when. They were called the Transfermium Working Group.
The TWG spent five years interviewing scientists at both labs and reviewing papers. They held weeklong meetings in seven different countries. Finally, they issued a report. They said that the hunt for new and fleeting elements was a difficult and complex task, interpreting the results even more so, and it was not always easy to say when discovery became a certainty. They thanked everyone for their work.
And then they said the Russians (and by now, they were Russians, not Soviets) had discovered element 102, nobelium, in, confusingly enough, 1966. Elements 103, 104, and 105 were ties. Berkeley was awarded credit for element 106, which Berkeley had named after its team member, the legendary nuclear chemist and Nobel laureate Glenn Seaborg.
Berkeley’s Ghiorso and Seaborg were appalled. The response they sent to the International Union’s newsletter said use by “the supposedly impartial TWG” of unpublished Russian papers and “retrospective reasoning” was “highly irregular” and “manifestly unfair.”
The next year the TWG issued its naming recommendations, suggesting that the element 104 should be named after the location of the Russian lab in Dubna, making it dubnium. And they said that no element should be named after a living person—no seaborgium.
Berkeley and others howled that Seaborg had, as the Bulletin of the Atomic Scientists put it, “disqualified himself by failing to expire.” They pointed out that no such objections had been made against either Albert Einstein (99, einsteinium) or Enrico Fermi (100, fermium).
Eventually in 1997, the International Union issued its final pronouncement. Element 106 would be seaborgium. In a shuffle, element 105 became dubnium. (Poor element 105 has had five different names—Berkeley partisans still call it hahnium.)
East–West tensions have since eased. These days, scientists in Dubna collaborate with scientists at the Lawrence Livermore National Laboratory. Together they have bragging rights to two elements: flerovium (114) and livermorium (116).
From the Spring 2014 Branding issue of California.